Nutraceutis and FSMPs
Current Pharmaceutical Biotechnology 2020 presents a review on current prospective of nutraceuticals. Nutraceuticals are dietary supplements, dietary fiber utilized to ameliorate health, delay senescence, prevent diseases, and support proper functioning of human body. Currently nutraceuticals are getting substantial attention due to nutrition and therapeutic potentials. On the basis of their source, they are categories into different terms such as nutrients, dietary supplements, herbals, dietary fiber, etc. Global market for nutraceutical is huge i.e approximately USD 117 billion. Herbal nutraceutical is a powerful tool in maintaining health and to act against nutritionally induced diseases, thus promoting optimal health, longevity, and quality of life. Studies have shown promising results of nutraceuticals to treat several diseases such as cancer, neurodegenative diseases, cardiovascular diseases, etc.
As reported by the British Journal of Clinical Pharmacology in 2018 nutraceuticals do not have a specific definition distinct from those of other food-derived categories, such as food supplements, herbal products, pre- and probiotics, functional foods, and fortified foods. Many studies have led to an understanding of the potential mechanisms of action of pharmaceutically active components contained in food that may improve health and reduce the risk of pathological conditions while enhancing overall well-being. Nevertheless, there is a lack of clear information and, often, the claimed health benefits may not be properly substantiated by safety and efficacy information or in vitro and in vivo data, which can induce false expectations and miss the target for a product to be effective, as claimed. An officially shared and accepted definition of nutraceuticals is still missing. A growing demand exists for nutraceuticals, which seem to reside in the grey area between pharmaceuticals and food. Nonetheless, given specific legislation from different countries, nutraceuticals are experiencing challenges with safety and health claim substantiation.
In this scenario, over its long experience in the field of clinical trials, OPIS has acquired the necessary tools to successfully manage clinical trials related to food supplements and Foods for Special Medical Purposes (FSMP). As they are clearly different from drug trials, food trials are currently regulated under the Food Supplements Directive (FSD) 2002/46/EC.
OPIS services for food trials are:
- Preparation or review of the protocol to ensure compliance with GCP guidelines.
- Evaluation of the study design and outcomes to verify they meet the regulatory requirements.
- Preparation of the necessary documentation (Investigator’s Brochures, Informed Consent Forms, and Case Report Forms).
- Support in the pre- or post-market authorization process.
- Proper trial execution.
- Compliant data handling procedures and statistical analysis.
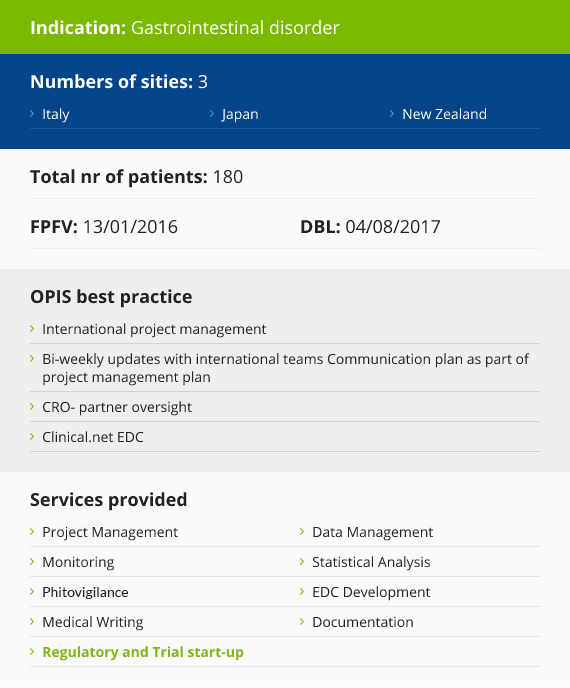
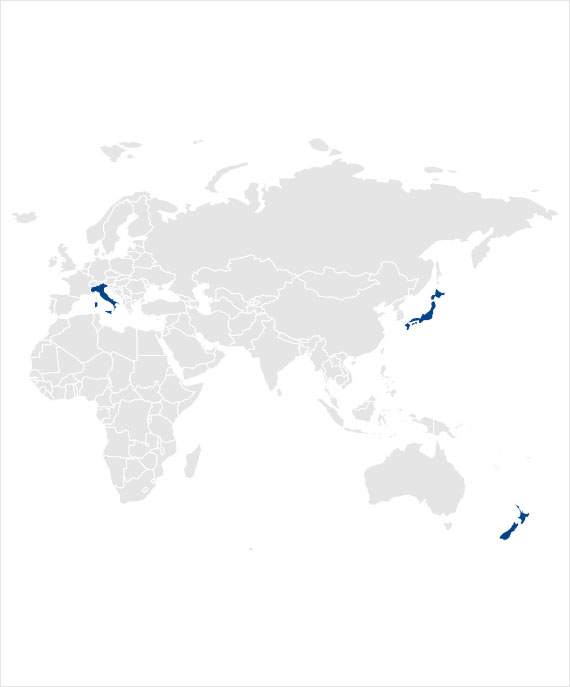
Case Study
Food for Special Medical Purpose Experience
Contact Us!
Whether you are Sponsor, a Clinical Research Professional or an Investigator contact us to learn more about how we can help.




