Infectious Diseases
Technological, industrial and biomedical developments have increased life quality and expectancy of the population, resulting in an increase in the world’s population, an increase in the number and speed of movements between continents and the development of new migration flows. The continuous evolution of human activities and changes of the global ecosystem has led to a necessary and parallel development of the pathogenic microorganisms, that continue to pose a challenge for medicine and pharmaceutical research.
The International Journal of Occupational and Environmental Medicine in 2017 highlights the correlation between global warming and health impact. Climate change scenarios include a change in distribution of infectious diseases with warming and changes in outbreaks associated with weather extreme events. After floods, increased cases of leptospirosis, campylobacter infections and cryptosporidiosis are reported. Global warming affects water heating, rising the transmission of water-borne pathogens. Pathogens transmitted by vectors are particularly sensitive to climate change because they spend a good part of their life cycle in a cold-blooded host invertebrate whose temperature is similar to the environment. A warmer climate presents more favourable conditions for the survival and the completion of the life cycle of the vector, going as far as to speed it up as in the case of mosquitoes. Diseases transmitted by mosquitoes include some of the most widespread worldwide illnesses such as malaria and viral diseases. Tick-borne diseases have increased in the past years in cold regions, because rising temperatures accelerate the cycle of development, the production of eggs, and the density and distribution of the tick population.
As a consequence, infectious diseases continue to spread rapidly posing a threat to public health triggered by the emergence of new unknown pathogens and the re-emergence of old infectious diseases. According to the World Health Organization infectious diseases are a leading cause of death worldwide particularly in developing countries and especially in young children. In addition, millions of people are developing cancers as a direct result of preventable infections by bacteria and viruses.
The increasing need to find effective treatments and vaccines for antibiotic resistant infections and highly contagious infectious diseases remain high priority in the face of improving global health. However, the multifaceted nature of developing adequate treatments is bringing elevated complexity to the process and conducting infectious disease clinical trials requires medical knowledge and above all, thorough understanding of the disease, its geography, seasonal behaviour, epidemiological manifestation and prevalence and incidence.
At OPIS, expert teams with proven methodology assist clients to overcome posed challenges and to streamline their clinical development.
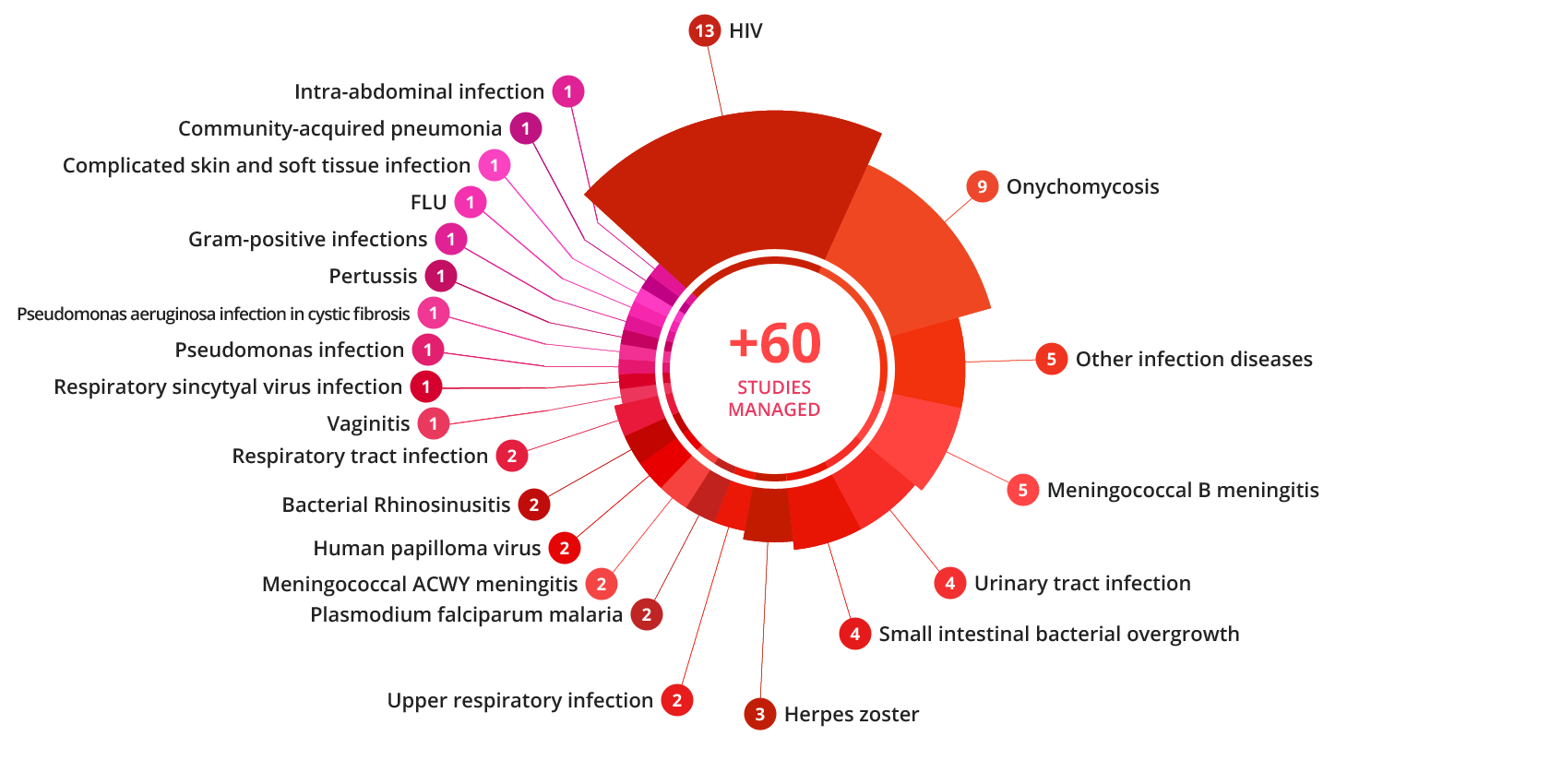
OPIS has managed 65 infectious disease trials across a wide range of indications and with various antibacterial, antiviral, antifungal, antiparasitic and vaccines treatments.
We have run more than 20 antiviral studies for HIV, herpes zoster, HPV, and more than 30 antibacterial studies including meningitis, urinary infections, skin and soft tissue infections, intra-abdominal infections, pseudomonas infections, among others.
OPIS experience in infectious diseases covers all phases of development (I-IV), clinical investigations for medical devices and studies with food supplements.
Solutions
- Our global investigator network of infectious diseases specialists enables us to identify the highest performing clinical sites and access to more patients meeting enrolment expectations, especially in case of seasonal diseases.
The strong relationship with investigator and regulatory experts, as well as our therapeutic experience and knowledge of disease prevalence and incidence, ensure an efficient and strategic execution of infectious disease clinical trials. - OPIS Medical Affairs Department provides protocol development consulting to ensure well-planned and efficiently conducted infectious disease clinical trials.
- Broad experience designing and conducting phase I-IV clinical trials in antibacterial, antiviral, antifungal, antiparasitic and vaccines treatments.
- Experience with pediatric populations.
Contact Us!
Whether you are Sponsor, a Clinical Research Professional or an Investigator contact us to learn more about how we can help.




