Advanced Therapy Medicinal Products
Advanced Therapy Medicinal Products (ATMPs) are medicines and therapeutic treatments that are based on genes, tissues or cells. They offer cutting-edge new opportunities for the treatment of various diseases in multiple therapeutic areas.
TMPs represent a fast-growing field of interest. The large number of ATMPs in development is likely to continue to grow, fuelled by a thousand of products in pilot studies.
Classification of ATMPs
- Gene therapy: drugs based on gene therapy technology that work by inserting recombinant genes into the body. A recombinant gene is a short string of synthetic DNA, carefully selected to correct the effect of a mutated gene that is causing the disease.
- Cell therapy: treatments based on cells or tissue that have been manipulated to change their biological characteristics and are used to cure, diagnose or even prevent diseases.
- Tissue-engineered medicines: these treatments contain cells or tissues that have been modified so they can be used to repair, regenerate or replace human tissue.
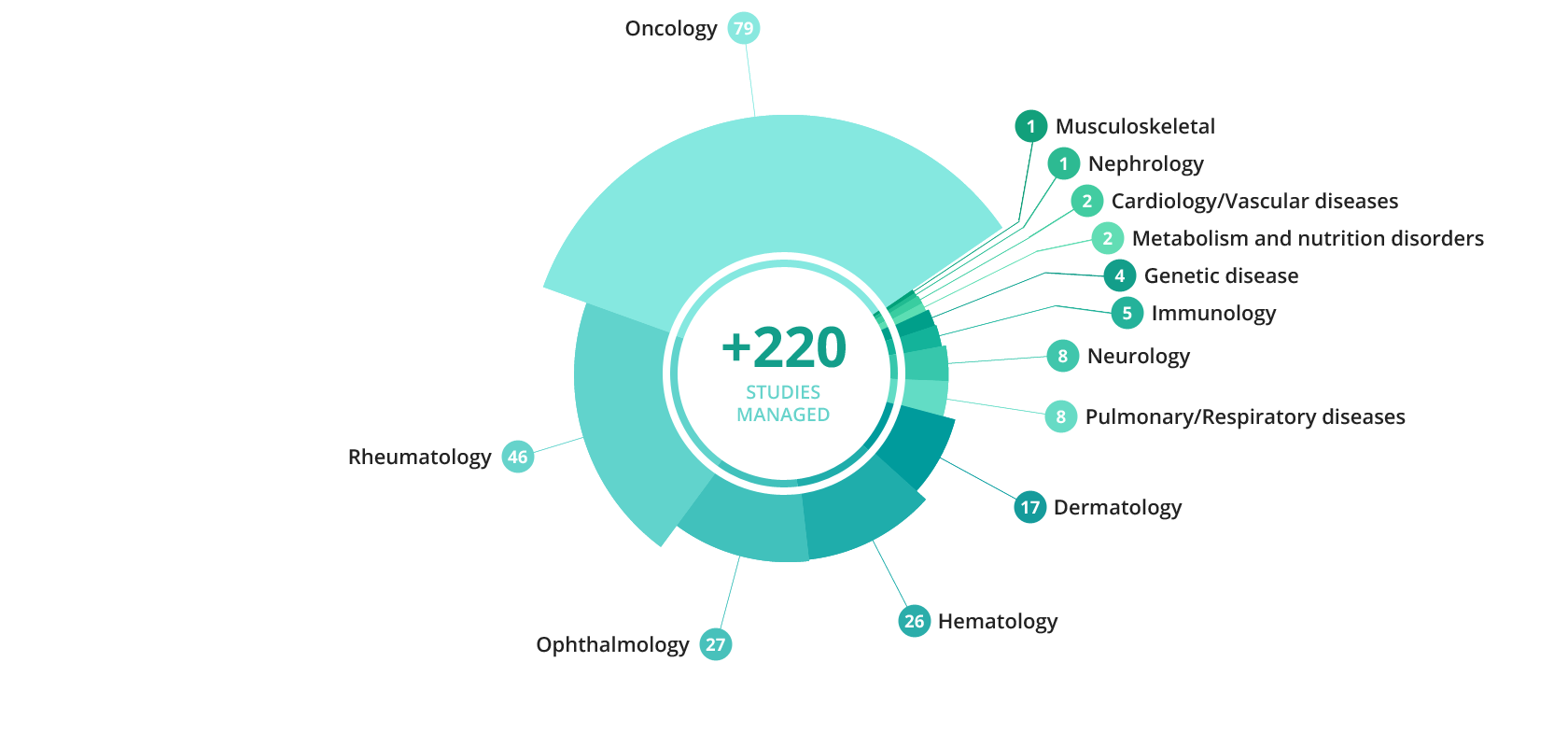
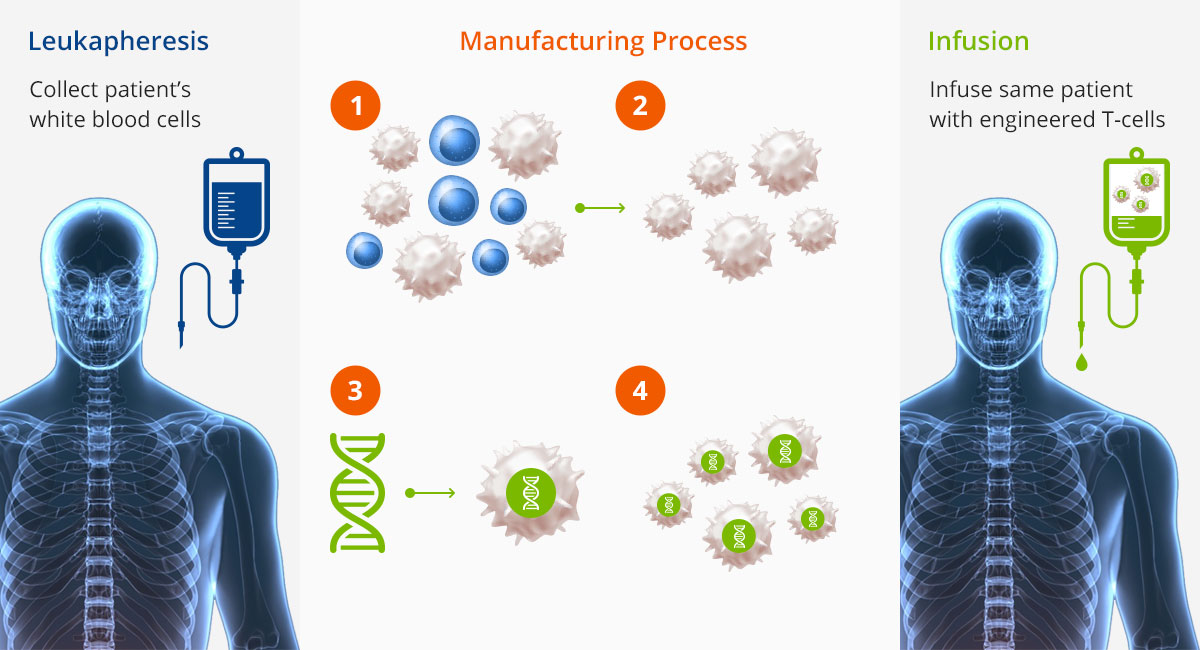
Cell Therapy Example: CAR-T CELL Process
Combined ATMPs
Some ATMPs may comprehend one or more medical devices as an integral part of the medicine, and these are referred to as combined ATMPs. A viable example of a combined ATMP is cells embedded in a biodegradable matrix or scaffold.
Action plan on ATMPs
As reported by Molecular Therapy Methods & Clinical Develepment in 2019, advanced therapy medicinal products (ATMPs) require evaluation by the European Medicines Agency’s Committee for Advanced Therapies prior to being placed on the European market, subject to a Marketing Authorisation granted by the European Commission. In common with other medicinal products, various regulatory pathways are available for taking ATMPs through clinical trials to market authorisation, and the regulatory pathway taken will depend on a product’s characteristics and the target patient population. With the industry poised to deliver more late-stage clinical and commercial ATMPs for serious diseases with high unmet medical need (e.g., T cell immunotherapies for cancer), bringing medicines to patients through optimized regulatory strategies and expedited pathways is assuming greater importance.
In 2018 EMA formed the Committee for Advanced Therapies (CAT) that plays a pivotal role in the scientific assessment of advanced therapy medicines; Indeed, it provides the necessary expertise to evaluate all proposed advanced therapy medicines from a scientific standpoint.
The CAT drafts a report and sends it to the Committee for Medicinal Products for Human Use (CHMP). Based on the CAT opinion, the CHMP may recommend or not the authorization of the treatment, which is eventually made by the European Commission.In 2016 the Journal of Market Access & Health Policy published a study aiming to characterise the ATMPs in development and discuss future implications in terms of market access. The paper highlghts that the development program (smaller sample sizes, single-arm trial designs, etc.) of such products may increase payers’ uncertainty about the product value at the time of launch. This uncertainty facing the payers and the important clinical benefits will contribute to the growing pressure put on payers during decision making. Coverage with evidence development with escrow agreements may become increasingly common in the pricing of ATMPs. The budget impact will possibly be considerable, threatening the sustainability of health insurance. The assessment of the expected clinical benefit, as well as the potential budget impact of several selected ATMPs, are the next steps of this project and have already been initiated.
Contact Us!
Whether you are Sponsor, a Clinical Research Professional or an Investigator contact us to learn more about how we can help.




